Oculogica Inc. is pleased to highlight a publication this week by a team of concussion researchers on the value of the EyeBOX concussion diagnostic technology in patients with persisting post-concussive symptoms. The study, Metrics of concussion-related vision disorders among children and adolescents with persisting post-concussive symptoms using an objective eye tracking device, was published in the Journal of Sport and Health Science on May 22, 2025. The study was led by Dr. Christina Master of Sports Medicine and Performance Center, Children’s Hospital of Philadelphia. The study team also included Dr. Mitchell Schieman (Drexel University), Dr. Olivia Podolak (Children’s Hospital of Philadelphia), Dr. Matthew Grady (Children’s Hospital Colorado Sports Medicine), and Dr. David Howell (University of Colorado).
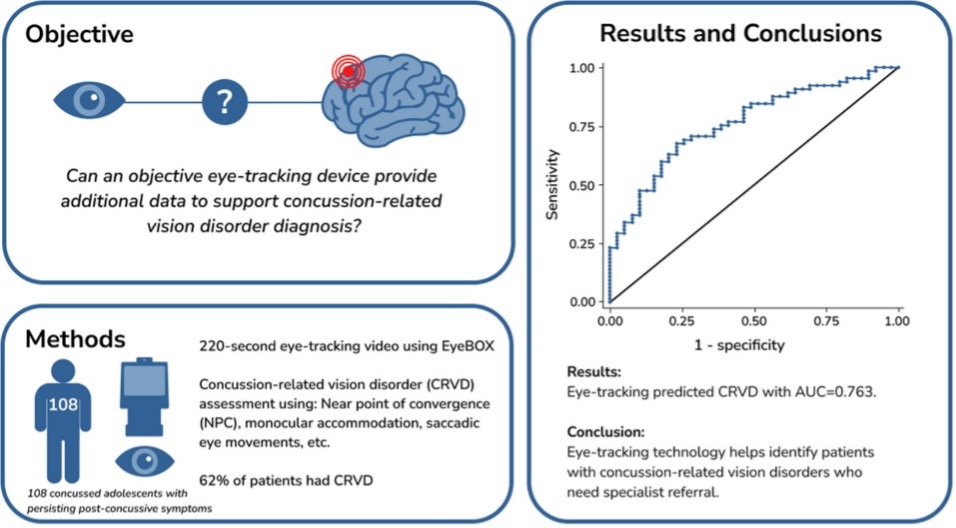
The study was designed to determine the utility of eye tracking in identifying concussion- related vision disorders (CRVD) in patients experiencing persistent post-concussive symptoms more than 28 days after injury. The objective was to determine if the EyeBOX could identify these patients accurately, as appropriate diagnosis and identification is critical for triaging patients to therapy.
The research included 108 concussed adolescents with persisting post-concussive symptoms who sustained a concussion 4-12 weeks prior to enrollment in the study. Sixty-seven of these patients (62%) were diagnosed with CRVD by comprehensive vision examination by a specialist. The EyeBOX successfully identified those with CRVD, and in particular for each 1-point increase in the BOX score (the EyeBOX score for concussion), the odds of the patient experiencing CRVD was found to be 15% higher. The study concluded that EyeBOX “may be a useful measurement in identification of those with concussion related vision disorder.”
“This is a major advancement for the field. Earlier, accurate diagnosis directly improves outcomes in concussed patients. Determining if a patient is at risk for concussion-related vision disorder quickly, and in patients who do not have immediate access to a specialist, is a major benefit for patients. We are thrilled with the publication and outcome of this study, which validates the value of our technology by a premier independent research group.” said Oculogica CEO Rosina Samadani, Ph.D.
The EyeBOX technology is the first non-invasive concussion diagnostic approved by the
FDA for aid in the diagnosis of concussion that does not require a pre-injury baseline
test. Leading clinics, such as the Mayo Clinic and Midwest Orthopedics at Rush University, as well as many local clinics such as Little Spurs Pediatric Urgent Care in Texas, offer the technology.
The full publication by Dr. Master, and other publications on Oculogica’s EyeBOX technology, are available here: https://oculogica.com/publications/.